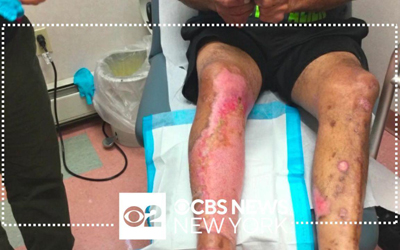
Vericel manufactures and markets two advanced cell therapy products for sports medicine and severe burn care in the United States and holds an exclusive license for North American commercial rights to NexoBrid®, a biological orphan product approved for eschar removal of severe thermal burns. Please use the links to the right to visit our product websites and learn more about our pipeline.




NASDAQ: VCEL
| $46.11 | -0.31 (0%) |
Day Low: $46.04
Volume: 199486
April 24, 2024